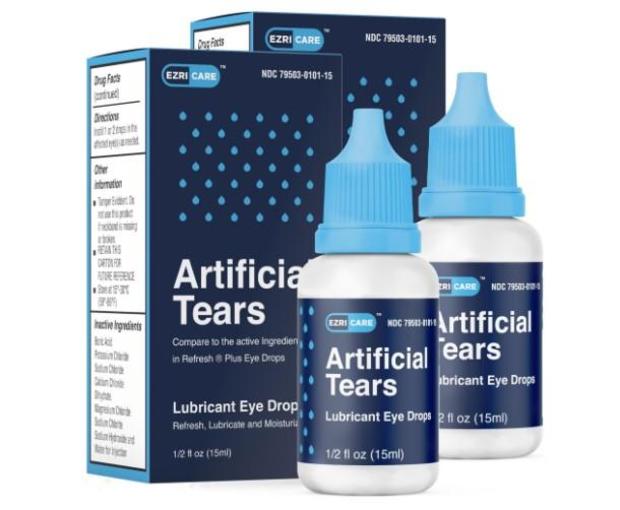
CDC says it will stop using Ezricare artificial tears as it investigates infections and deaths

The Centers for Disease Control and Prevention recommends that consumers and health care providers stop using EzriCare Artificial Tears immediately. That’s because the test looks at a “multistate cluster” of eye drop-related infections. At least one death has been linked to the infection.
CDC found infection in 50 patients in 11 states Saidadded that most of them use artificial tears, and the most commonly used brand is Ezricare.
Outcomes for some patients include “eye infections, hospitalizations and [the] One patient died from a bloodstream infection,” the CDC said, noting that bacterial infections are often resistant to antibiotics.
ezricare-info.com
The CDC said it “recommends that clinicians and patients immediately discontinue use of EzriCare artificial tears” until probes and laboratory tests are completed. Authorities are working with state and local health departments on this issue.
ezrikale he said We have not received any consumer complaints or reports of side effects related to the investigation, or word from any regulatory agency regarding the investigation, nor have we been asked to implement a recall.
Still, the company said, “out of caution,” customers should stop using “any portion of EzriCare Artificial Tear Lubricant Eye Drops” until more information is available about potential safety concerns. strongly emphasized.
Ezricare states that EzriCare Artificial Tears were “formulated, designed and imported by”. Aru Pharma Co., Ltd. manufactured by GLOBAL PHARMA HEALTHCARE PVT LTD.
Some law firms Simmons Hanley Conroy etc.state-owned enterprises are already looking for potential plaintiffs.
Thank you for visiting CBS News.
Create a free account or log in
for more features.
https://www.cbsnews.com/news/ezricare-artificial-tears-cdc-infections-death/ CDC says it will stop using Ezricare artificial tears as it investigates infections and deaths